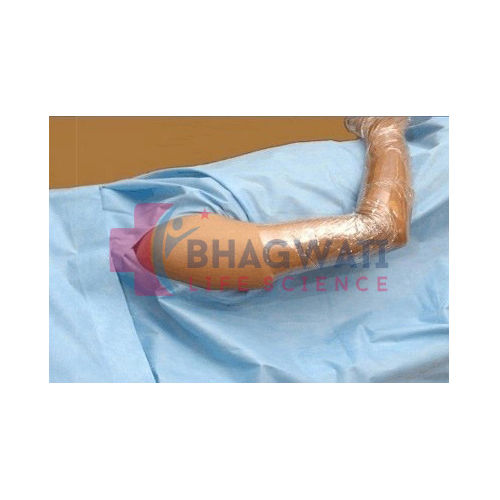
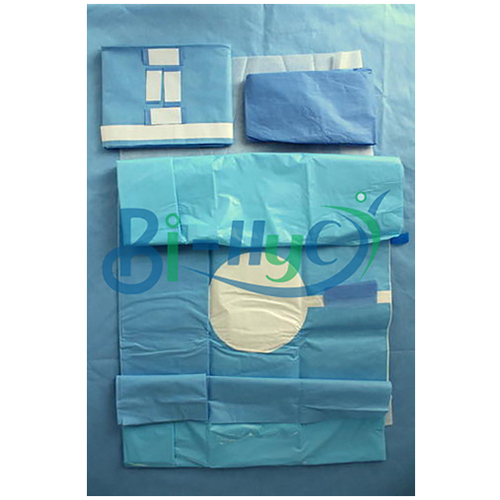
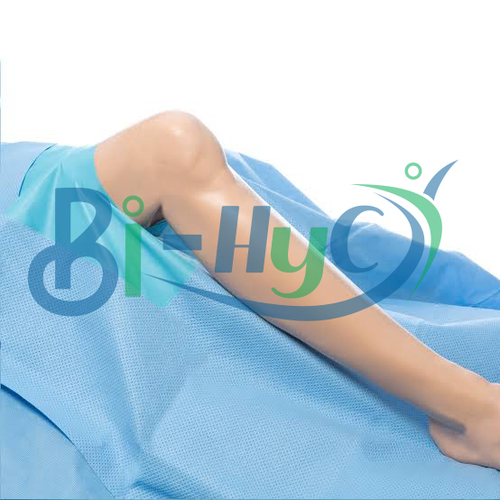
Arthroscopic Surgery Kit
Price 900 INR/ Piece
Arthroscopic Surgery Kit Specification
- Shelf Life
- 3 Years
- Storage Instructions
- Store in a cool, dry place
- Weight
- Lightweight
- Size
- Universal/Procedure Specific
- Usage & Applications
- Hospital, Arthroscopic Procedures
- Set Contains
- Drapes, Towels, Gown, Gloves, Wrap, Surgical Instruments
- Adhesive Type
- Non-Adhesive
- Dressing Type
- Surgical Kit
- Disposable Type
- Disposable
- Style
- Pre-packed Kit
- Cuff
- Yes
- Disposable
- Yes
- Recyclable
- No
- Sterilized
- ETO
- Waterproof
- Yes
- Shape
- Rectangular/Custom Fit
- Length
- Standard
- Width
- Standard
- Grade
- Medical Grade
- Packaging
- Individually sterile packed
- Suitable For
- Single Use Only
- Compliance
- ISO 13485, CE Marked
- Latex Free
- Yes
- OEM Availability
- Yes
- Tear Resistance
- Enhanced Durability
- Protection Level
- Fluid-resistant
- Absorbency
- High
- Instrument Tray Included
- Yes
- Application Method
- Ready to Use
Arthroscopic Surgery Kit Trade Information
- Minimum Order Quantity
- 50 Pieces
- Payment Terms
- Cash in Advance (CID)
- Supply Ability
- 100 Pieces Per Day
- Delivery Time
- 2 Days
- Sample Available
- Yes
- Sample Policy
- Free samples are available
- Certifications
- An ISO 9001 & 13485
About Arthroscopic Surgery Kit
Product details
| Type | Fixable Drapes |
| Material | SSMMS |
| Packaging | Sterile |
| Size | 320*240cm |
| Color | Medical Blue |
| Sterility | Sterile |
| Category | Reinforced |
| Usage/Application | Orthopaedic |
Comprehensive Surgical Solution
This arthroscopic surgery kit provides everything required for a sterile, efficient arthroscopic procedure, including drapes, gowns, gloves, and surgical instruments. Each item is fluid-resistant, offering optimal protection for both patient and surgical staff. Pre-packed and ready to use, the kit streamlines preparation and improves procedural workflow, making it an indispensable asset in hospital environments.
Strict Quality and Safety Compliance
Manufactured under ISO 13485 standards and CE Mark certified, the kit emphasizes medical safety and performance. ETO sterilization and fluid resistance are critical features, ensuring patient protection and procedural hygiene. With enhanced tear resistance, the kit components maintain durability throughout demanding arthroscopic applications.
Single-Use, Hassle-Free Design
The entire kit is latex-free, waterproof, and designed for one-time use, ensuring sterility for each procedure. Individually packed and lightweight, it minimizes the risk of cross-contamination while simplifying post-operative waste handling. Proper storage extends its shelf life up to three years.
FAQs of Arthroscopic Surgery Kit:
Q: How should the Arthroscopic Surgery Kit be used during surgical procedures?
A: The kit is designed for immediate use in arthroscopic procedures. Simply remove the sterile packaging and arrange the included itemsdrapes, gowns, gloves, towels, wrap, and instrumentsaccording to the surgical protocol. All components are ready to use, ensuring an efficient and safe set-up for every operation.Q: What are the benefits of using a fluid-resistant, high-absorbency kit for arthroscopy?
A: Fluid resistance and high absorbency help contain fluids during surgery, maintaining a drier and safer operating area. This reduces the risk of infections and enhances comfort for surgical staff, ensuring optimal outcomes in every arthroscopic procedure.Q: Where should the kit be stored before use to ensure maximum shelf life?
A: To maintain sterility and product integrity, store the kit in a cool, dry place away from direct sunlight or excessive moisture. This ensures its efficacy and extends its shelf life up to three years.Q: Is the kit suitable for patients with latex allergies?
A: Yes, the arthroscopic surgery kit is entirely latex-free, making it safe for use with patients and staff who have latex sensitivities or allergies.Q: What ensures the safety and quality compliance of this kit?
A: The kit is manufactured according to ISO 13485 standards and is CE Marked, indicating strict adherence to international medical safety and quality regulations. All its components undergo ETO sterilization and meet medical-grade material requirements.Q: When should this kit be disposed of after use?
A: As a single-use, disposable kit, it must be discarded immediately after the procedure according to hospital and medical waste guidelines. Reuse is not permitted to ensure infection control.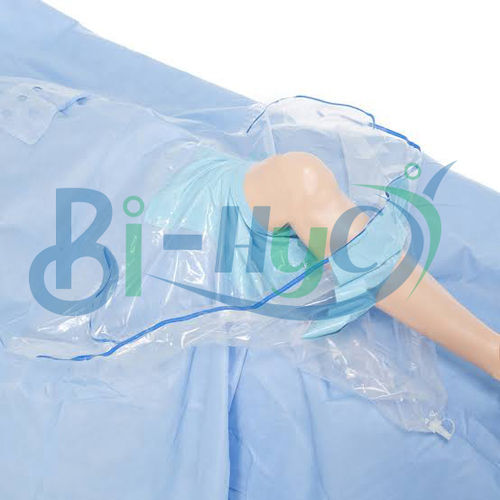

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Orthopedic Drape Category
TKR Kit
Price 1500 INR / Piece
Minimum Order Quantity : 50 Pieces
Waterproof : Yes
Disposable Type : Other, Single Use
Size : Universal
Dressing Type : Other, Surgical
Shoulder U Drape
Price 350 INR / Piece
Minimum Order Quantity : 50 Pieces
Waterproof : Yes
Disposable Type : Other, Single Use
Size : Universal, Covers Shoulder Area
Dressing Type : Other, Surgical Drape
THR Kit
Price 1500 INR / Piece
Minimum Order Quantity : 50 Pieces
Waterproof : Yes
Disposable Type : Other, Single Use
Size : Standard
Dressing Type : Other, Surgical Dressing Kit
Upper Extremity Drape
Price 550 INR / Piece
Minimum Order Quantity : 50 Pieces
Waterproof : Yes
Disposable Type : Other, Single Use
Size : Universal
Dressing Type : Other, Surgical Drape
 |
BHAGWATI LIFE SCIENCE
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese
 Send Inquiry
Send Inquiry