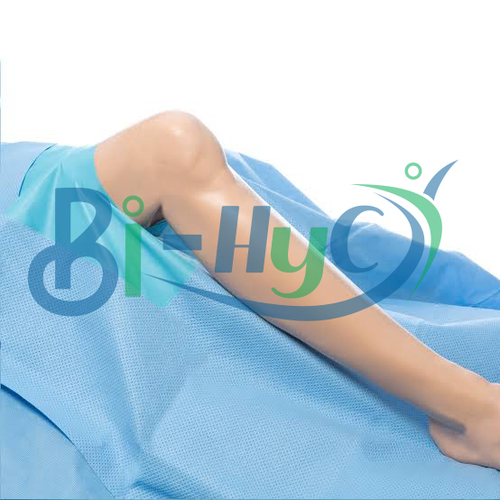
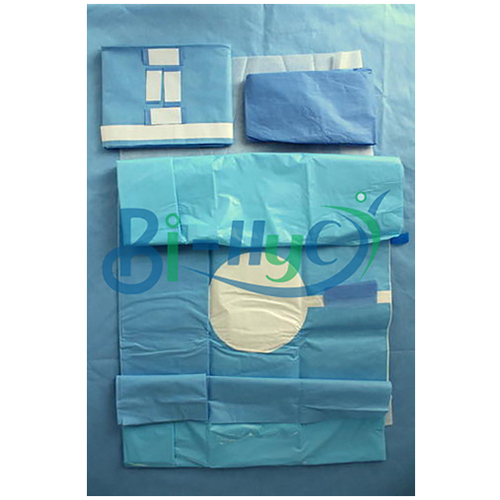
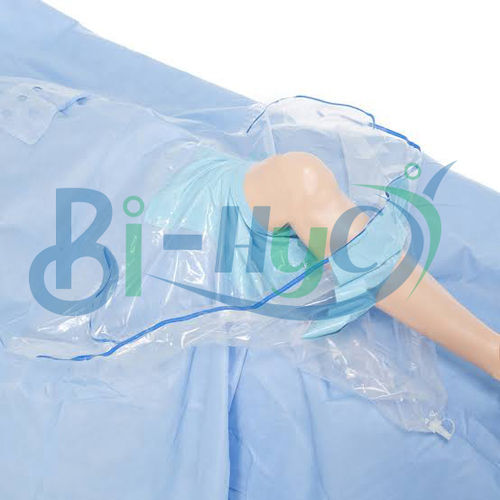
Major Drape
Price 340 INR/ Piece
Major Drape Specification
- Usage & Applications
- Used as a sterile field to cover patients during major surgical procedures
- Size
- Standard and Custom Sizes Available
- Adhesive Type
- No adhesive
- Weight
- Lightweight
- Set Contains
- Single Piece per Pack
- Storage Instructions
- Store in a cool, dry place away from direct sunlight
- Shelf Life
- 5 Years (Unopened)
- Dressing Type
- Surgical Drape
- Disposable Type
- Single Use
- Style
- Plain/Fenestrated (as per requirement)
- Cuff
- Elastic Cuff for secure fit
- Disposable
- Yes
- Recyclable
- No
- Sterilized
- Yes, ETO Sterilized
- Waterproof
- Yes
- Shape
- Rectangular
- Length
- 200 cm (approx.)
- Width
- 160 cm (approx.)
- Grade
- Medical Grade
- Barrier Performance
- Advanced microbial and fluid barrier
- Edge Finish
- Ultrasonically Sealed Edges
- Transparency
- Opaque
- Tear Resistance
- Enhanced for surgical safety
- Manufactured By
- Renowned Indian Manufacturer
- Compatibility
- Safe for use with ETO and Steam sterilization
- Latex-Free
- Yes (100% latex free)
- Packing Type
- Sterile Packaging, Peelable Pouch
- Compliance Standard
- CE & ISO 13485 Certified
- Fluid Repellency
- High
Major Drape Trade Information
- Minimum Order Quantity
- 50 Pieces
- Supply Ability
- 100 Pieces Per Day
- Delivery Time
- 2 Days
- Sample Available
- Yes
- Sample Policy
- Free samples are available
- Main Export Market(s)
- Australia, North America, South America, Eastern Europe, Western Europe, Middle East, Africa, Central America, Asia
- Certifications
- An ISO 9001 & 13485
About Major Drape
Product details
| Country of Origin | Made in India |
| Type | Fixable Drapes |
| Material | SSMMS |
| Packaging | Sterile |
| Size | 160*300cm |
| Brand | Bi-HyC |
| Color | Medical Blue |
| Sterility | Sterile |
| Category | Standard |
| Usage/Application | Orthopaedic |
Advanced Barrier Protection
The Major Drape features high fluid repellency and an advanced microbial barrier, making it ideal for demanding surgical environments. Its SSMMS material ensures both patient and staff remain protected from fluid strike-through and potential contaminants throughout the procedure, supporting an optimal sterile field.
Designed for Surgical Safety
Enhanced tear resistance and ultrasonically sealed edges provide robust integrity, minimizing risk during surgery. The elastic cuff ensures a secure fit, and the latex-free composition reduces allergen exposure. Each drape undergoes ETO sterilization and is packaged in a peelable sterile pouch, maintaining aseptic conditions.
Flexible, Convenient, and Compliant
Available in both plain and fenestrated variants, and in standard or custom sizes, this drape accommodates diverse surgical needs. It meets international safety and quality standards, carrying CE and ISO 13485 certifications. Lightweight yet strong, it is single-use, aligning with best-practice infection control protocols.
FAQs of Major Drape:
Q: How should the Major Surgical Drape be used during surgery?
A: The drape is intended to create a sterile barrier over the patient in major surgical procedures. Remove it from its sterile, peelable pouch, and position it over the surgical area. Ensure the elastic cuff is secured for optimal coverage, and use the plain or fenestrated style as appropriate for the procedure.Q: What benefits does a latex-free, SSMMS drape provide?
A: Being 100% latex-free eliminates the risk of allergic reactions for both patients and staff. The SSMMS material ensures excellent fluid repellency and an advanced microbial barrier, maintaining a sterile operative field and reducing infection risk.Q: When should I use a sterile, disposable major drape?
A: Sterile, single-use major drapes are necessary for all major and invasive surgical interventions where maintaining a high level of sterility and protection is critical. Always check the packaging is unopened and the drape is within its five-year shelf life before using.Q: Where can these drapes be stored safely?
A: Store the drapes in a cool, dry location away from direct sunlight to maintain the integrity of the sterile barrier and prevent material degradation. Proper storage conditions help ensure the drapes remain effective until use.Q: What certifications and compliance standards does this drape meet?
A: This surgical drape complies with CE and ISO 13485 certification standards, confirming its safety, quality, and effectiveness for use in medical and surgical settings worldwide.Q: What makes this surgical drape resistant to tearing?
A: Enhanced tear resistance is achieved by using robust SSMMS material and ultrasonically sealed edges, which bolster the drapes strength during handling and throughout surgical procedures.Q: Is the drape compatible with all sterilization methods?
A: The drape is specifically designed for ETO and steam sterilization, ensuring compatibility with standard hospital sterilization processes. It is supplied ETO sterilized and ready for use.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Orthopedic Drape Category
Upper Extremity Drape
Price 550 INR / Piece
Minimum Order Quantity : 50 Pieces
Disposable Type : Other, Single Use
Weight : Approx. 120 grams
Cuff : Other, Provided for secure grip
Color : Medical Blue
THR Kit
Price 1500 INR / Piece
Minimum Order Quantity : 50 Pieces
Disposable Type : Other, Single Use
Weight : Varies as per kit contents
Cuff : Other, Elastic Cuff
Color : Medical Blue
Arthroscopic Surgery Kit
Price 900 INR / Piece
Minimum Order Quantity : 50 Pieces
Disposable Type : Other, Disposable
Weight : Lightweight
Cuff : Yes
Color : Medical Blue
TKR Kit
Price 1500 INR / Piece
Minimum Order Quantity : 50 Pieces
Disposable Type : Other, Single Use
Weight : Approx. 1.2 kg
Cuff : Other, Double Cuff
Color : Medical Blue
 |
BHAGWATI LIFE SCIENCE
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese
 Send Inquiry
Send Inquiry